Difference between revisions of "Interpretation of NMR Results"
| Line 5: | Line 5: | ||
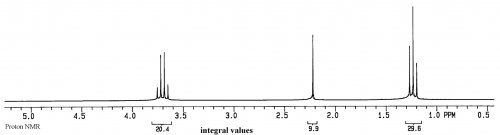
If a sample has just a single compound with a concentration of 1-10mg/ml, a proton NMR spectrum will look similar to: | If a sample has just a single compound with a concentration of 1-10mg/ml, a proton NMR spectrum will look similar to: | ||
| − | This spectrum is of ethanol. | + | [[File:proton NMR of ethanol.png|500px]] |
| + | |||
| + | This spectrum is of ethanol. The Y axis is relative intensity, while the X axis is relative frequency. Instead of using Hz for frequency, for historical reasons the unit is called ppm, or parts per million, and the scale begins at 0 from the right side and increases in ppm to the left. | ||
==Peaks== | ==Peaks== | ||
Revision as of 16:11, 30 April 2020
Contents
Introduction
The main element that is studied in NMR is hydrogen. In the literature hydrogen is called proton, hence the name proton NMR. A proton NMR spectrum will only show protons, no other elements. Similarly, a carbon spectrum will show only carbons. The other major nuclei that can be studied with NMR are nitrogen, phosphorus and fluorine. Each nucleus requires special tuning of the instrument to be able to see.
What does the result look like?
If a sample has just a single compound with a concentration of 1-10mg/ml, a proton NMR spectrum will look similar to:
This spectrum is of ethanol. The Y axis is relative intensity, while the X axis is relative frequency. Instead of using Hz for frequency, for historical reasons the unit is called ppm, or parts per million, and the scale begins at 0 from the right side and increases in ppm to the left.